Tracking the metrics of scientific innovation in the midst of a global pandemic
Interview with Peter Revill, Clarivate Analytics
Subscribe: Apple Podcasts | Stitcher | Email | Download
 With nearly 373,000 cases of COVID-19 confirmed worldwide, and more than 16,000 deaths to date resulting from the coronavirus pandemic (as of 24 March 2020, according to the WHO), scientists around the world are attempting to develop vaccines and treatments as quickly as possible.
With nearly 373,000 cases of COVID-19 confirmed worldwide, and more than 16,000 deaths to date resulting from the coronavirus pandemic (as of 24 March 2020, according to the WHO), scientists around the world are attempting to develop vaccines and treatments as quickly as possible.
Recent examples of the increasing activity to tackle this disease include a collaboration between the Gates Foundation, Wellcome Trust, and MasterCard, to establish $125 million seed fund to accelerate development of drugs that treat COVID-19, as well as the increasing number of biopharma companies with drug development activities against COVID-19.
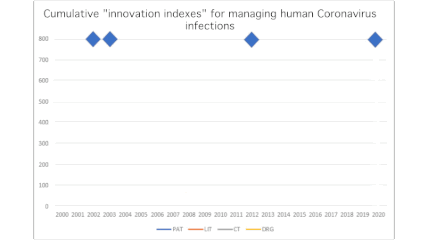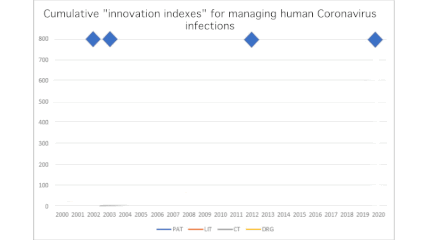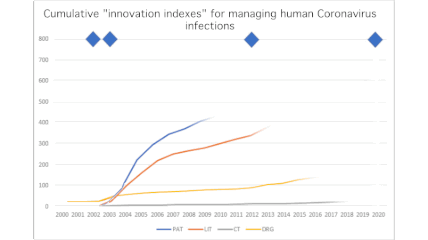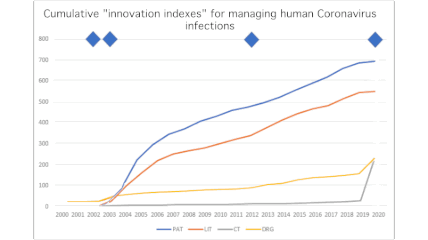 Peter Revill, a Life Sciences product manager at Clarivate Analytics, is tracking the metrics of scientific innovation in the midst of our pandemic. When he spoke last Friday with CCC’s Chris Kenneally, Revill contrasted the COVID-19 research explosion with activity that followed the MERS and SARS outbreaks in the recent past.
Peter Revill, a Life Sciences product manager at Clarivate Analytics, is tracking the metrics of scientific innovation in the midst of our pandemic. When he spoke last Friday with CCC’s Chris Kenneally, Revill contrasted the COVID-19 research explosion with activity that followed the MERS and SARS outbreaks in the recent past.
“There’s a huge difference as we come to the current pandemic,” Revill explains. “Immediately in the first two months of this year we’ve seen a great flurry of activity around those drug milestone events. Large numbers of organizations are really staking their claim and saying that we have these compounds, and we’re taking them into development as potential vaccines or cures for this disease.
“Most remarkably of all, [there are already even] clinical trials. So that’s a real statement of intention to develop a treatment. We’ve seen, for example, over the years, typically around about two, three, four new clinical trials a year since 2016. In 2019, we saw five new clinical trials added. In the first two months of this year, 244 new clinical trials. And now we’re up at 470.
Peter Revill is currently coordinating Clarivate’s Coronavirus resources initiative, part of a wider initiative in the biomedical and healthcare publisher community to provide free access to Coronavirus data to healthcare researchers and medical professionals in support of their efforts to better understand and combat COVID-19.
Earlier this month, he wrote about his observations for Copyright Clearance Center’s Velocity of Content blog.

